It is a pair of point charges with equal magnitude and opposite in sign separated by a distance. A water molecule is an example of an electric dipole. Even though the water molecule as such is neutral, the chemical bond within the molecule causes displacement of charges. This displacement of charges creates a dipole with a negative charge on the oxygen end and a net positive charge on the hydrogen end.
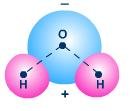
If water molecules were not electric dipole, water would have been poor solvent.
Related Articles: